Rapid Test Kit Cov-19 Antigen Saliva Rapid Test Kit High
Specificity 1 Test Professional Antigen Saliva Rapid Test Kit
Intend Use
SARS-CoV-2 antigen saliva rapid test kit is intended for detection
SARS-CoV-2 antigen in human saliva sample. The test kit is an
qualitative detection rapid test kit for professional use. About
the test result, the positive result can be used forearly triage
and rapid management of suspected populations, further nucleic acid
detection should be carried out for suspected population whose
antigen test result is positive or negative.
Product Details
| Item | Value |
| Name | SARS-CoV-2 Antigen Saliva Rapid Test Kit |
| Model Number | LX-401601 |
| Type | 1 Tests/Kit |
| Warranty | 18 Months |
| Quality Certification | CE, MSDS |
| Safty Standard | ISO13485 |
| Sample Type | Saliva |
| Sample volume | 3 Full drops |
| Test Speed | Within 10-15 minutes |
| Specificity | 99.00% |
| Sensitivity | 94.29% |
| Total Accuracy | >95% |

Product Advantage
- Short test time, read the result within 10-15 mins
- Excellent performance, Strong Test line and Control line
- Lower cost with high effciency
- Reliable safety without extra instument
- More comfortable, convenience and protable

Main Components
- 1 Test Cassettes
- 1 Saliva Collectors With Collection Tubes
- 1 Sample Extraction Buffer
- 1 Instruction Manual

Use Step
- Open the cap of the collection tube and istall saliva collector.
- Put the collection tube with saliva collector close to lips and let
the saliva flow into the collection tube. Tje volum of saliva needs
to be half of 0.5 scal mark(0.25).
- Screw the sample extraction buffer carefully.
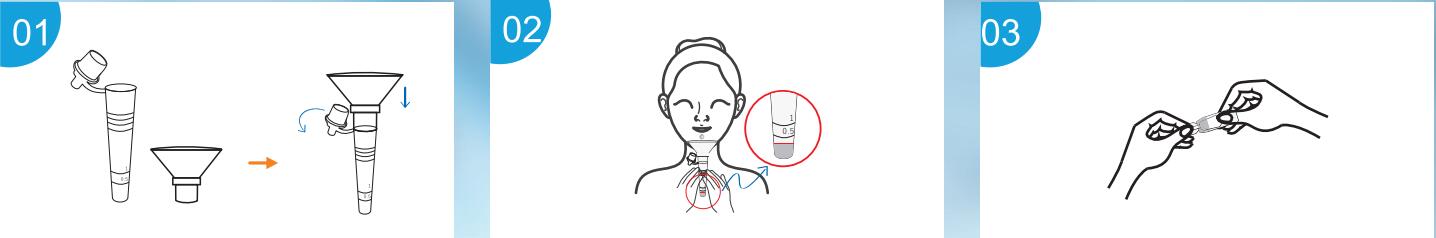
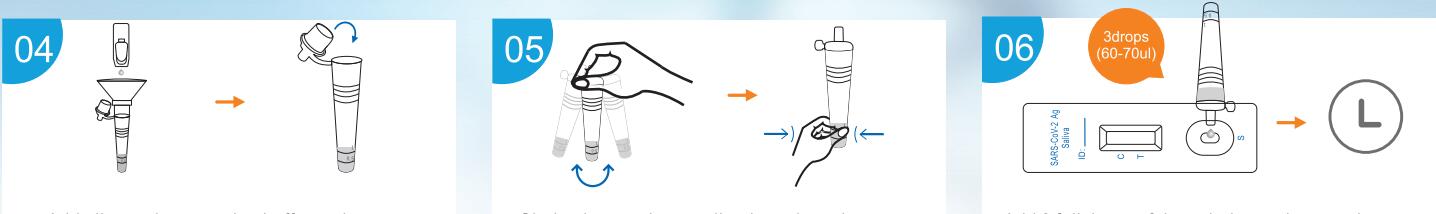
- Add all sample extration buffer to the collection tube. Discard the
saliva collector, tighten the cap onto the specimen collection
tube.
- Shake the specimen collection tube at least three time vigorously
to mix the saliva and the extraction buffer. Squeeze bottom of the
collection tube ensure the saliva is thoroughly mixed.
- Add 3 full drops of the solution to the sample well(S). Read the
result at 15 minutes. Do not interpret the result after 20 minutes.
Result Interpretation
POSITIVE: Two (2) distinct colored lines appear. One line should be in the
control region (C) and the other line should be in the test region
(T).
NEGATIVE: One (1) colored line appears in the control region(C). No apparent
colored line appears in the test region (T). The negative result
does not indicate the absence of analytes in the sample, it only
indicates the level of tested analytes in the sample is less than
the minimum detection limit.
INVALID: No colored lines appear, or control line fails to appear,
indicating that the operator error or reagent failure. Verify the
test procedure and repeat the test with a new testing device.

Principle
- This kit is an immunochromatography assay. According to the gold
immunochromatographic test principle, double antibody sandwich
method was used to detect SARS-CoV-2 nucleocapsid antigen in the
samples. If there is antigen in the sample, complex can be formed
at the detection line, and red band is shown, indicating negative
result. If there is no antigen in the sample, complex cannot be
formed at the detection line, and no red band is shown, indicating
negative result.
- Whether the sample contains antigen or not, the gold monoclonal
antibody will bind to the enveloped antibody at the quality control
line, form a compound and condense into a red band.
Application
- Center for Disease Control and Prevention
- Primary Health Care Institution
- School
- Prison / Detention Center etc.
Virus Sources
| Global high frequency mutation | Alpha / B.1.1.7(U.K.) | Beta I B.1.351(South Africa) |
| Gemma I P.1(Brazil) | Kappa I B.1.617.1(India) | Delta I B.1.617.2(India) |
| C.37,ect | Alpha I B.1.17(U.K.) | B.1.36.16.etc |
| A.2.5,etc | A.23.1 | Alpha I B.1.17(U.K.) |
| B.1.1.33.etc | C.1.1.etc. | etc. |
Stroage And Expiry
Store as packaged in the sealed pouch at 2-30℃, avoid heat and
sunshine, dry place, valid for 24 months. Do not freeze. Do not
open the inner packaging until ready, it must be used within one
hour if opened (Humidity≤60%, Temp: 20℃-30℃). Please use
immediately when the humidity>60%.
Certificate

FAQ
We have the MOQ limit, which is 10000 pieces.
Air Cargo, Ocean Cargo or by Express
Business to Business account
Whatsapp, E-mail or Phone.
After order confirmed, we will arrange your order immediately,and
offer you an estimated delivery date.
OEM Or ODM Accept
Yes, We accept OEM / ODM order.















