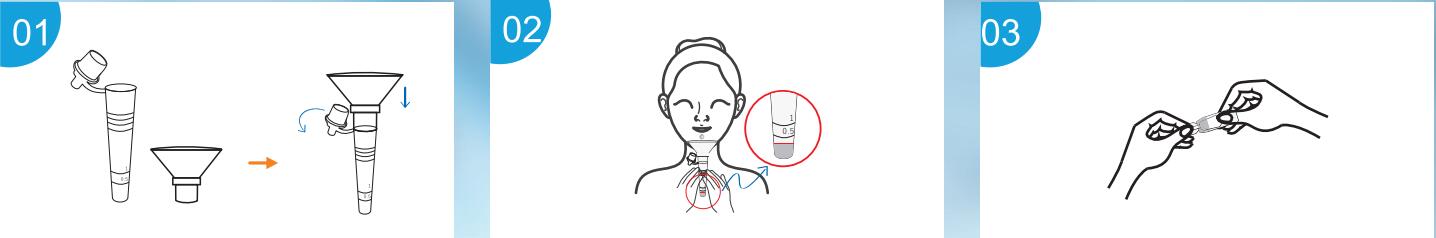
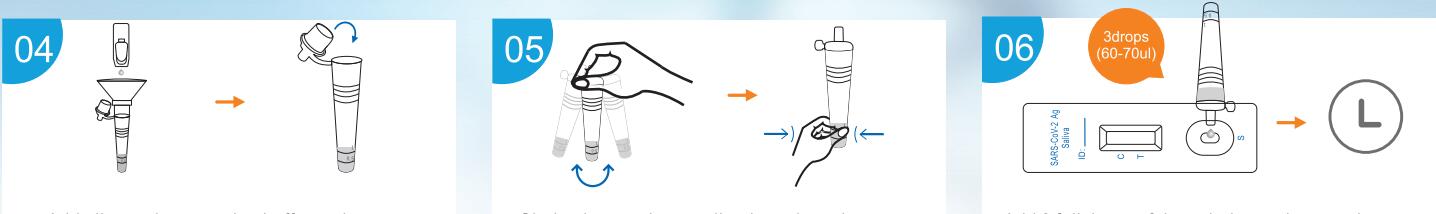
Labnovation Technologies, Inc.
Labnovation Technologies, Inc. is an original manufacturer
established in Shenzhen, China since 2001. We are dedicated in
developing and manufacturing IVD instruments and reagents including
HbA1c analyzer and reagents, rapid test reader & reagents,
hematology reagents, clinical chemistry reagents, electrolyte
reagents, wash solution and urine test reagents.
Labnovation products are developed in house, tested extensively in
clinics and passed strict quality controls. All of our products are
CE marked and approved by SFDA. In the past 15 years, our products
have been exported to 110 different countries and used in 8000
hospitals worldwide. We provide both ready-to-use final products
and bulk OEM reagents, including ready-to-use reagents,
concentrated, and powder.
With an investment of 10% of our annual sales into R&D, we
release new products every years. We win more and more domestic and international cooperation by
company reputation and products quality while we actively take part
in fairs like MEDICA,AACC, ARBA HEALTH and so on through which
we are well-known by the companies in this field.